Water, a seemingly simple molecule, holds profound significance in the realms of chemistry, biology, and environmental science. Its molecular structure, consisting of two hydrogen atoms covalently bonded to one oxygen atom (H₂O), belies the complexity of its behavior and unique physico-chemical properties. Understanding the intricacies of water is pivotal, considering its omnipresence and fundamental role in sustaining life on Earth.
1. Molecular Structure:
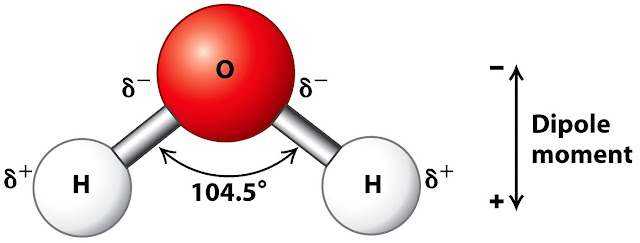
- Chemical
Composition: Water (H₂O) is a polar molecule consisting of two
hydrogen atoms covalently bonded to one oxygen atom.
- Polarity:
The oxygen atom is more electronegative, creating a polar molecule with a
partial negative charge near the oxygen and partial positive charges near
the hydrogen atoms.
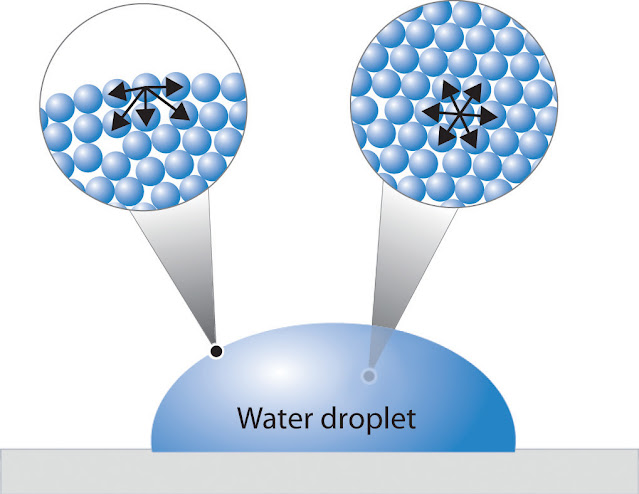
2. Hydrogen Bonding:
- Intermolecular
Forces: Water molecules exhibit hydrogen bonding due to the attraction
between the partially negative oxygen of one molecule and the partially
positive hydrogen of another.
- Unique
Properties: Hydrogen bonding contributes to water's unique properties,
including high surface tension, cohesion, and adhesion.
3. States of Matter:
- Solid
(Ice): In the solid state, water molecules form a hexagonal lattice
structure, with each molecule bonded to four others through hydrogen
bonds.
- Liquid:
In the liquid state, water molecules are in constant motion, and hydrogen
bonds are dynamic.
- Gas
(Vapor): In the gaseous state, water molecules have high kinetic
energy and are widely separated.
4. Density Anomalies:
- Maximum
Density at 4°C: Unlike most substances, water reaches its maximum
density at 4°C, causing ice to float on liquid water.
- Expansion
upon Freezing: Water expands when it freezes due to the arrangement of
its molecules in a hexagonal lattice.
5. High Heat Capacity:
- Temperature
Regulation: Water has a high specific heat capacity, allowing it to
absorb and retain large amounts of heat. This property regulates
temperature in both living organisms and the environment.
6. Solvent Properties:
- Universal
Solvent: Water is often referred to as the "universal
solvent" because it can dissolve a wide range of substances,
facilitating chemical reactions and supporting life processes.
- Hydration:
Ions and polar molecules are surrounded by water molecules in a process
called hydration.
7. Surface Tension:
- Cohesive
Forces: Water molecules at the surface experience an inward pull due
to hydrogen bonding with neighboring molecules, resulting in surface
tension.
- Capillary
Action: Surface tension contributes to capillary action, allowing
water to move against gravity in narrow spaces.
8. pH and Ionization:
- Neutral
pH: Pure water is neutral, with a pH of 7. It can ionize into hydrogen
ions (H⁺) and hydroxide ions (OH⁻).
- Acid-Base
Properties: Water participates in acid-base reactions and acts as a
buffer, resisting drastic changes in pH.
9. Transparency and Absorption:
- Visible
Light Transmission: Water is transparent to visible light, allowing
sunlight to penetrate aquatic environments.
- Selective
Absorption: Water selectively absorbs different wavelengths,
influencing the color spectrum observed in aquatic systems.
10. Chemical Reactivity:
- Hydrolysis: Water participates in hydrolysis reactions, breaking down complex molecules into simpler ones through the addition of water molecules.
- Ionization of Salts: Water can ionize salts into their constituent ions, influencing the pH of aqueous solutions.
Understanding the detailed structure and physico-chemical properties of water is fundamental to various scientific disciplines, including chemistry, biology, and environmental science. The unique characteristics of water contribute to its essential role in supporting life and shaping natural processes.
FAQs
- What
is the molecular structure of water, and why is it considered a polar
molecule?
- Answer:
Water has a molecular structure of H₂O, with a polar nature due to the
unequal sharing of electrons between oxygen and hydrogen, resulting in
partial positive and negative charges.
- How
does hydrogen bonding contribute to the unique properties of water?
- Answer:
Hydrogen bonding, formed between the hydrogen of one water molecule and
the oxygen of another, imparts properties like high cohesion, surface
tension, and the ability to act as a solvent.
- Why
does water have a maximum density at 4°C, and how does it impact aquatic
ecosystems?
- Answer:
Water reaches its maximum density at 4°C, causing ice to float. This
property is crucial for the survival of aquatic life, preventing bodies
of water from freezing entirely.
- What
is the significance of water's high specific heat capacity in biological
systems?
- Answer:
Water's high specific heat capacity allows it to absorb and retain heat,
regulating temperature in biological systems and maintaining stable
environmental conditions.
- How
does water act as a solvent, and why is it called the 'universal solvent'?
- Answer:
Water's polarity enables it to dissolve a wide range of substances,
making it the 'universal solvent' and facilitating chemical reactions in
biological and environmental processes.
- What
role does water play in biological systems, and how does it support life?
- Answer:
Water is essential for life as it serves as a medium for biochemical
reactions, facilitates nutrient transport in organisms, and maintains
cellular structures.
- Why
is water transparent to visible light, and how does this property
influence aquatic ecosystems?
- Answer:
Water's transparency to visible light allows sunlight to penetrate
aquatic environments, influencing photosynthesis and the distribution of
life in water bodies.
- How
does water contribute to the regulation of pH and participate in acid-base
reactions?
- Answer:
Water can ionize into hydrogen ions (H⁺) and hydroxide ions (OH⁻),
playing a role in pH regulation and participating in acid-base reactions.
- What
are the environmental implications of water's selective absorption of
different wavelengths of light?
- Answer:
Water's selective absorption influences the color spectrum in aquatic
environments, impacting the availability of light for photosynthesis and
affecting ecosystems.
- In
what ways does water contribute to environmental processes, such as
weathering and nutrient cycling?
- Answer:
Water participates in processes like hydrolysis, influencing weathering
of rocks and contributing to nutrient cycling by transporting and
dissolving minerals in the environment.


0 Comments